Vaccine storage

The history of vaccines is a great example of how science, medicine, and technology have all improved over time. Immunization has always been an important part of controlling and sometimes even getting rid of infectious diseases. For example, smallpox vaccination started in the late 1700s and COVID-19 vaccines were developed very quickly. As vaccines became more complex and sensitive, it became clear that their effectiveness depended not only on how they were made but also on how they were stored, moved, and used.
As vaccines became more advanced, it became clear that proper storage and management of the cold chain were very important. Early medical practitioners understood that inadequate storage conditions could jeopardize vaccine integrity, resulting in diminished efficacy or total loss of protective attributes. This understanding led to the creation of specialized storage equipment, standardized protocols, and regulatory frameworks to keep vaccines effective from the time they are made until they are given. Today, storing vaccines is seen as an important part of immunization programs all over the world.
Problems Associated with Improper Storage
If vaccines are not stored properly, they can lose their effectiveness, which is the most serious consequence. When vaccines are stored in places that are too hot, too humid, or too bright, the active ingredients can break down, making them less effective or even completely ineffective. It is often impossible to see such degradation, which means that vaccines that have been compromised may still be given without anyone knowing.
The effects go beyond losing money from wasted doses. Vaccines that don't work can leave people with weak immunity, making them more likely to get diseases that could have been avoided, and they may need to get vaccinated again, which puts more stress on healthcare systems. On a larger scale, storage problems can make people less trusting of vaccination programs and put public health at risk. Some common mistakes when storing things are exposing them to temperatures that are too high or too low, too much humidity, not enough protection from light, and not handling them properly when they are being moved or used regularly.
Principles of Vaccine Storage
Effects of Temperature on Traditional Vaccines
Traditional vaccines, such as those for polio, measles, and influenza, require storage and transportation at a controlled temperature range of +2°C to +8°C to maintain efficacy. These vaccines are sensitive to heat; exposure to temperatures above +8°C can cause cumulative and progressive degradation of the active ingredients. Although these vaccines can withstand room temperature for a few days during manufacturing and administration, higher temperatures accelerate this degradation process.
Vaccines Stored at Very Low Temperatures
Emerging vaccines, including viral vector vaccines like the Ebola vaccine and mRNA vaccines, require storage at much lower temperatures, specifically -90°C to -60°C and -25°C to -15°C. These vaccines are less stable and more fragile, necessitating strict temperature controls to ensure their efficacy. The COVID-19 vaccines from BioNTech Pfizer and Moderna exemplify this, with specified shelf lives at these low temperatures. This shift to extreme cold storage poses challenges for the existing logistics chains, which were previously not designed for such conditions.
The Refrigerant Challenge in Cold Chain Logistics
Temperature-controlled logistics for ultra-low temperatures is crucial. The industry faces significant challenges in using high Global Warming Potential (GWP) refrigerants below -50°C, such as the commonly used R404A and R452A in transport refrigeration units. Replacing R23 with natural refrigerants offers an interesting alternative for these extreme conditions, though considerations for energy efficiency and safety remain paramount. However, Air-based refrigeration has proved higher efficiency and safety, while keeping running costs to a minimum.
Promising options include natural refrigerants like carbon dioxide (CO₂) and hydrocarbons, as well as air, which is increasingly recognized as a viable refrigeration medium. The subsequent table highlights the environmental impacts and hazard levels of these alternatives compared to conventional refrigerants.
|
Refrigerants |
Chemical Name |
GWP (100 years) |
Safety Class |
Application |
|
R22 |
Chlorodifluoromethane |
1780 |
A1 |
-20℃ |
|
R23 |
Fluoroform |
12690 |
A1 |
-70℃ |
|
R134a |
1,1,1,2- Tetrafluoroethane |
1360 |
A1 |
0℃ |
|
R170 |
Ethane |
1.4 |
A3 |
-70℃ |
|
R600 |
Butane |
<1 |
A3 |
-20℃ |
|
R718 |
Water |
- |
A1 |
0℃ |
|
R729 |
Air |
- |
A1 |
-70℃ |
Humidity
Temperature control is still the most important thing to think about when storing vaccines, but humidity control is also very important. If there is too much humidity, condensation can form inside storage units. This can damage packaging, make labels less sticky, and hide important information like expiration dates or batch numbers. These problems make it more likely that mistakes will happen when handling things, and they can also mess up inventory management.
Protection from Light
A lot of vaccines are sensitive to light and can break down when they are in direct or long-term light. Vaccines should be kept in containers that block light or in opaque packaging to keep them from getting damaged by light. Storage units and handling procedures should be set up so that people don't have to be exposed to things they don't need to be.
Types of Refrigerated Storage Devices
Refrigerated vaccine storage includes purpose-built vaccine refrigerators, pharmaceutical-grade units, and freezer units for vaccines requiring storage at ultra-low temperatures. These devices often come with integrated temperature monitoring and alarm systems to alert healthcare professionals of any deviations from the required storage conditions.
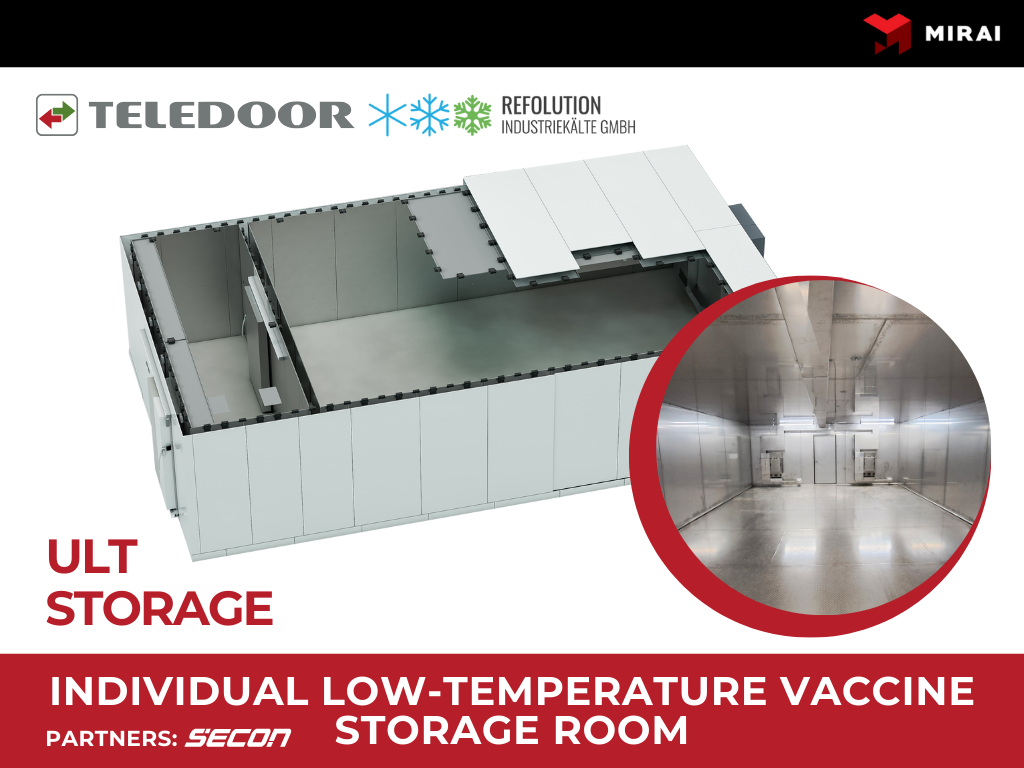
MIRAI Products that can be Used for Vaccine Storage
MIRAI's range of pharmaceutical-grade refrigeration machines offers precise temperature control, making them ideal for the application of vaccine storage. These refrigeration machines are designed to maintain uniform temperature conditions and have built-in data logging features for monitoring and management of vaccine storage conditions.Mirai ULT Space coolers are equipped with a humidity extraction device - Snow Catcher, a revolutionary Humidity Extraction Device (HED) which traps the humidity from the air in the chamber and extracts it mechanically. Snow Catcher is equipped with a Snow Tray that collects captured moisture, transforms it into snow, and thus helps prevent ice formation or snow accumulation on storage elements. The defrost procedures are not required.

In addition, a nice benefit is that the equipment is completely environmentally friendly and produces no emissions. To achieve such low air temperatures, Mirai uses its engineered turbo-module in its machines, which does not need regular service and can guarantee reliability to its customers.
Mirai Intex machines are a favorite among leaders in the pharmaceutical industry. It has extensive experience in projects for vaccine storage and biomaterials. Projects allowed for publication can be found on the website in the references section, where it is also possible to see a project with KTI mobile storage container and Thomaidis CryoCargo - mobile vaccine storage.
When it comes to reliability, temperature control and energy efficiency, there is no substitute. In addition, when it comes to equipment usability, Mirai has an excellent competitive advantage - customization to suit customer changes. The customer does not need to change something in the machine or buy a new machine, if they need on a permanent or temporary basis a different temperature than originally designed, they simply set the necessary settings on the touch screen and the machine works according to them.
Requirements for Vaccine Storage
The safe storage and handling of vaccines are paramount in maintaining their efficacy and ensuring public health safety. The CDC's (Centre for Disease Control) guidelines outline the requirements for vaccine storage, including:
- Approved Equipment. Only CDC-approved refrigeration units should be used for storing vaccines to ensure they meet the necessary temperature and humidity specifications.
- Management and Handling Protocols. Facilities must follow strict protocols for the management and handling of vaccines, including regular training for staff and maintaining detailed records of storage conditions.
- Temperature Monitoring. Continuous monitoring of vaccine storage temperature is essential. Digital data loggers with detachable probes are recommended for accurate temperature readings.
- Transport. Vaccines must be transported in validated coolers with required temperature range.
- VFC Compliance. Facilities participating in the Vaccines for Children (VFC) program must adhere to specific storage requirements, including having backup storage plans in case of power outages or equipment failures.
The implementation of a comprehensive vaccine storage and handling protocol, including a proper checklist and the use of approved refrigeration equipment, is crucial for the safe and effective management of vaccines. By adhering to these guidelines, healthcare providers can ensure that vaccines retain their potency and are safe for administration to the public, thereby protecting communities from preventable diseases.