Reliable Blood Plasma Storage: Key Risks and Solutions

Problems with Plasma Storage and Solutions
In the critical infrastructure of modern medicine and biopharmaceutical production, frozen plasma storage of blood plasma ranks among those monumental medical and technological challenges that have not diminished in importance relative to advances in technology, critical treatment, or medical care in general. Plasma itself is not a commodity—it is a biologically active substance that is potentially one's lifeline, consisting of proteins, enzymes, and clotting factors that are highly sensitive, necessary, and therefore judicious in plasma quality preservation. It is a key part of a huge logistical network that includes blood banks, hospital transfusion services, and fractionation plants, all of which are necessary for life.
When this temperature-controlled chain is broken, the inherent plasma storage problems happen, and important factors like Factor VIII are permanently damaged. This damage is not usually caused by sudden accidents. The results are bad. Patients are directly harmed by lower therapeutic efficacy, and financial losses from spoiled batches threaten operations. Regulatory non-compliance with bodies like the FDA risks punitive action. In the end, these failures come together, making the plasma storage supply chain less safe for patients and less trustworthy.
To solve blood plasma problems that are all connected, we need to go beyond basic refrigeration. It requires a full understanding of the thermodynamic, mechanical, and regulatory risks, as well as the use of precision-engineered plasma storage solutions to ensure uncompromising reliability and true plasma quality preservation from vein to vial.
The Importance of Appropriate Plasma Storage
The need for strict control over blood plasma storage arises from the inherent instability of its biological substances. Plasma proteins are extremely sensitive to changes in temperatures. All major health regulating authorities across the globe, from the FDA, EDQM, and the WHO, require strict control over the temperatures of plasma during its storage is often maintained at -25°C and below for long-term preservation, with extensions to -40°C and -80°C for specific purposes.
Clinically, improper units of blood plasma storage renders them ineffective and potentially dangerous, as it may trigger adverse reactions and fail to provide the necessary hemostatic effect. A temperature change can cause all plasma stocks to be quarantined, which can break supply chains for hospitals and fractionators, leading to shortages and higher costs. Because of this, proper storage is very important for both plasma therapy production and transfusion services.
Essential Conditions for Blood Plasma Storage
The most important and non-negotiable requirement is to keep the plasma storage temperature very low and stable for decades. This strict thermal requirement is necessary because plasma proteins are unstable. If the temperature excursions, it can start a chain reaction of molecular breakdown that makes it harder to keep the quality of the plasma. For true effectiveness, you need an engineered environment that goes far beyond a simple "cold box."
It needs a validated frozen plasma storage system, which means that every part has been tested and shown to work well in the worst possible situation. Calibrated sensors must be used at all important load points, not just the air return, for strong, ongoing monitoring. For regulatory audit trails, tamper-evident data logging is a must because it makes an unchangeable custody record. Redundancy built into important parts, like two refrigeration circuits, helps avoid single points of failure. Finally, strict documentation rules for IQ, OQ, PQ, and preventative maintenance turn a regular appliance into a qualified pharmaceutical cold storage.
The main goal is to make sure that things stay stable over time. In this context, stability means more than just temperature. It also means that the system can work with consistent mechanical and thermal reliability, recover quickly from power outages or door openings without major temperature excursions, and provide consistent ultra-low temperature storage throughout the chamber.
Temperature Uniformity and Stability
Getting the temperature to the right level is only half the battle; it's also important to make sure that the temperature is evenly spread throughout the storage space. When air doesn't move around well or the design is bad, temperature gradients form. These create "hot spots" where plasma can partially thaw and "cold spots" that waste energy. This means that identical units stored together can degrade at very different rates, which makes the overall inventory less safe and consistent.
Ice Crystal Formation and Freezing Speed
The freezing process is as important as the food's long-term storage temperature or pharma lyophilization. This is because the size and shape of the ice crystals that form in the plasma matrix depend directly on the thermodynamic path taken during the phase change. Slow freezing occurs when a blood plasma storage unit lacks sufficient cooling power or fails to transfer heat well, allowing water molecules plenty of time to move around and group together. They break cell membranes and cut delicate plasma proteins like fibrinogen and Factor VIII, which permanently damages their biological activity and clotting ability.
To keep the plasma quality at its best, the freezing phase must be carefully controlled as a key point in the process. The goal is to move plasma units from above freezing to the target plasma storage temperature as quickly and evenly as possible so that they spend as little time as possible in the "critical zone" between -1°C and -30°C, where the most harmful crystal growth happens. This principle is supported by empirical data; comparative testing of freezing performance, like the one done by MIRAI INTEX, shows how different technologies work in very different ways. The graphs below show the results of the tests. MIRAI INTEX plasma freezing systems with advanced air-cycle refrigeration show a much faster pull-down time and better chamber uniformity than regular ultra-low temperature storage freezers. This performance advantage has a direct effect on the final quality and therapeutic yield of the frozen plasma.
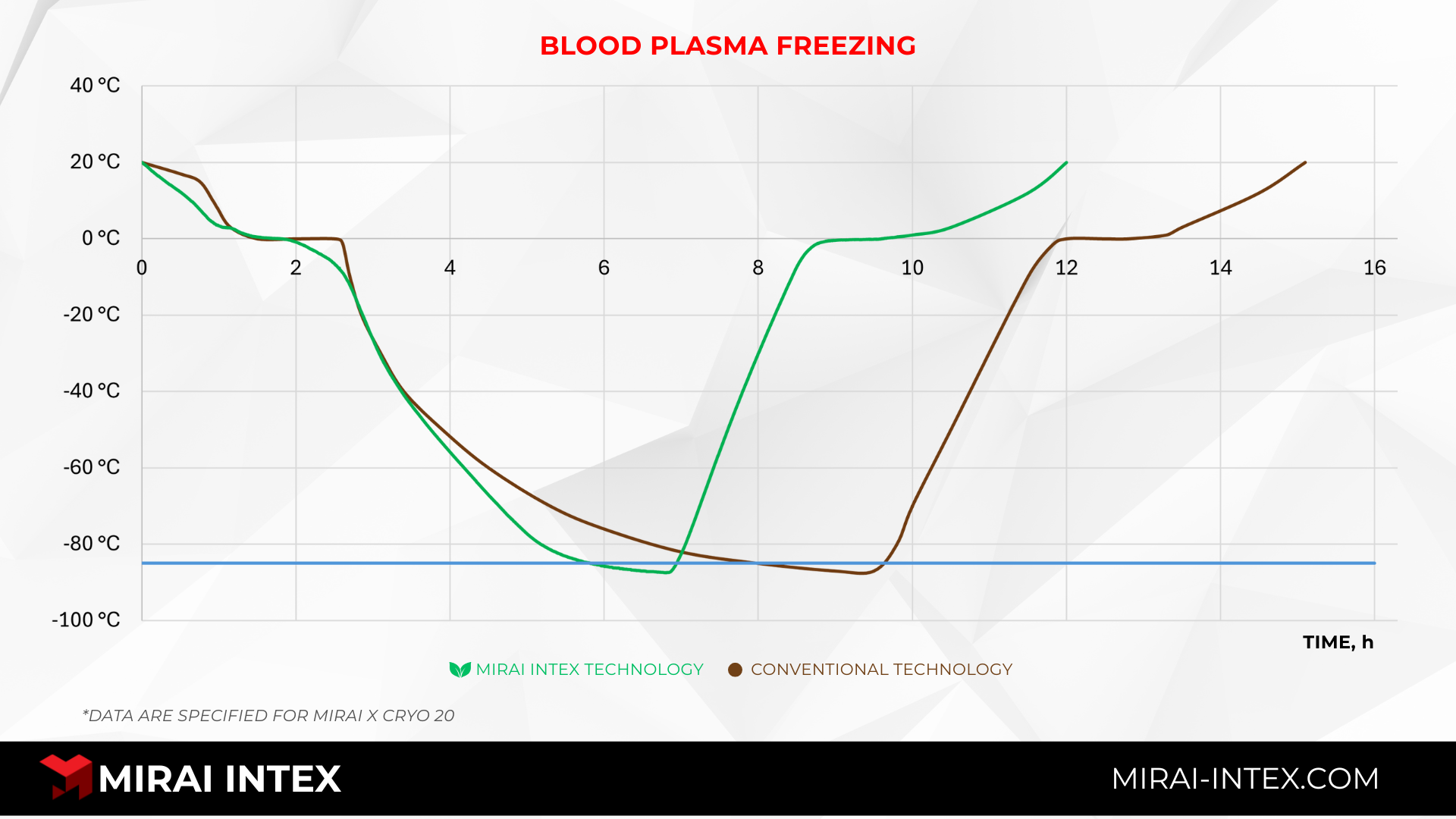
Typical Issues with Plasma Storage
Even though there are clear rules for storing plasma, problems are common, and they are often caused by the limitations of traditional equipment. These problems puts the system's integrity and the plasma's viability at risk. The most common problems are temperature excursions, bad freezing processes, problems with managing ice, risks related to refrigerants, and costs that can't be sustained. Each of these problems is connected to others and needs a specific technological solution.
Variations in Temperature While Being Stored
Stability is very important, but many traditional medical cold storage units are not very stable. There are always small changes because of compressor cycling, periodic defrosting, and recovery from door openings. Over time, these repeated changes in temperature break down plasma proteins. More serious deviations can happen because of mechanical failures, power outages, or slow recovery, which can put inventory safety at risk.
Freezing That Is Slow or Uneven
If a system doesn't have enough cooling power or bad thermal transfer, it will freeze slowly or unevenly. This makes for a mixed product because some plasma units freeze correctly while others do not, which makes the quality inconsistent. This kind of plasma might not be good for sensitive pharmaceutical processing or might have a shelf life that is hard to predict.
Cycles of Ice Accumulation and Defrost
In vapor-compression systems that use standard refrigerants, moisture from the air around the system always gets into the storage chamber and builds up on the evaporator coils as frost. This ice works as an insulator, which makes it much less efficient at transferring heat. The system then has to start a defrost cycle that uses a lot of energy, where heaters warm the coils and melt the ice. This process makes the chamber very hot, which puts the product at risk of being exposed to high plasma storage temperature spikes. It also causes downtime when temperature control is not working.
Risks Associated with Refrigerants
Hydrofluorocarbons (HFCs) like R23 or CO₂ or hydrocarbons are used in many high-performance systems. These materials could leak, which would lower their cooling power, be toxic, or catch fire. Also, they have to deal with global phase-downs because of rules like the EU F-Gas regulation. This means that equipment with certain refrigerants is a stranded asset. Maintaining these materials is more difficult and expensive because only certified workers can do it.
High Downtime and Maintenance Costs
Frequent defrost cycles, regular maintenance to keep the refrigerant charge and ice buildup under control, and the wear and tear on compressors and heaters all cause more downtime. This maintenance is not only expensive, but it also makes the system vulnerable at times when the reliability of plasma storage depends on manual intervention and system recovery. That is the place where oil-free compressor technology is needed.
How to Prevent Issues with Plasma Storage
To stop plasma storage problems, you need to change the way you think about them. The main reason is usually not a mistake in how things are done but a lack of technology. So, the answer is to choose storage systems that are built from the ground up to get rid of these failure modes, with a focus on uniform freezing, temperature stability, and long-term use.
Ensuring Uniform and Quick Freezing
The freezing stage is where prevention begins. To make sure that all plasma units quickly and evenly pass through the critical phase change, technology must be able to extract heat in a powerful and directed way. For this to work, the airflow design has to be very advanced and the freezing ramp rate has to be very precise. The end result is that small ice crystals form evenly throughout the product, which is what makes it stable for a long time when stored at very low temperatures.
Removing Temperature Variations
To get rid of the built-in instability of defrost cycles, we need a paradigm shift. The best system keeps a cold space that is completely sealed off from moisture. Without moisture, coils don't freeze, so there is no need for defrost cycles. This architectural method makes sure that the temperature stays stable all the time, which means that plasma won't degrade over time due to temperature excursions. It also makes sure that the system can quickly recover from outside disruptions.
Selecting Future-Proof and Safe Refrigeration
The choice of refrigeration technology has a direct effect on how long it will last. Air-cycle refrigeration technology is a future-proof option that only uses clean, dry air as its working fluid. It gets rid of the risks that come with refrigerants, such as leaks, toxicity, and flammability. It also automatically follows changing environmental rules, ensuring that the system for storing blood plasma remains in good working order and compliant for its entire service life.
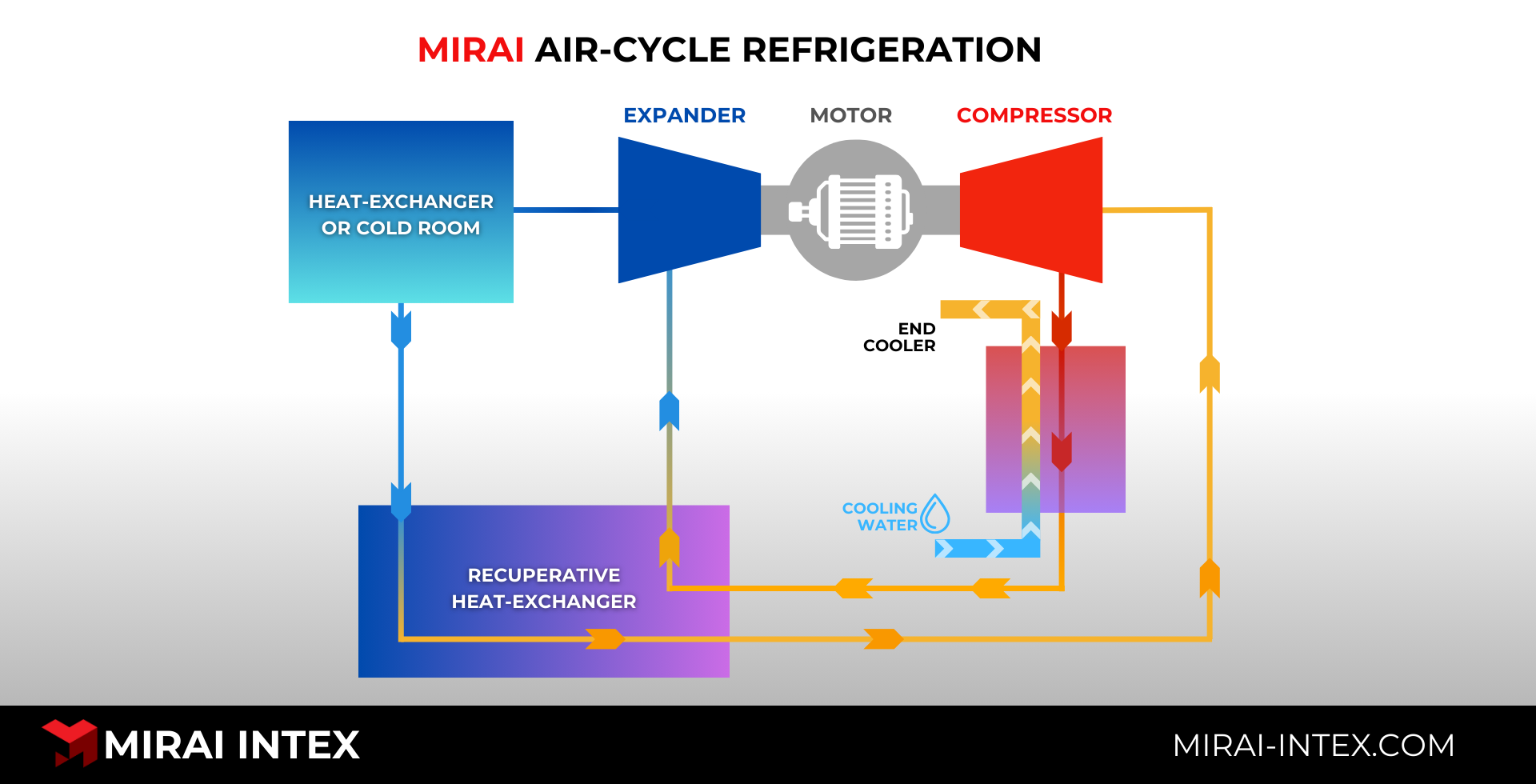
Advanced Refrigeration Technologies' Role
Modern plasma storage solutions are using advanced thermodynamic cycles to get around the problems that come with old-fashioned compressors. These technologies change the way cooling works at a basic level, putting stability, sustainability, and reliability ahead of just getting the temperature right. These solutions directly address the main plasma storage issues of ice formation, temperature excursions, and reliance on refrigerants by focusing on system architecture, such as making closed-loop environments or using different compression mediums.
Why Air-Cycle Technology Addresses Various Problems
Air-cycle refrigeration is based on a simple and beautiful thermodynamic cause-and-effect principle. It completely changes how we store things at very low temperatures. It addresses the fundamental issues with conventional plasma storage by utilizing a closed, hermetically sealed cycle that exclusively utilizes clean, dry, and inert air as its refrigerant. In a traditional system, moisture from the air always gets into the storage chamber, which causes frost to build up. Because the air-cycle refrigeration system is sealed, even with open-cycle refrigeration systems this moisture can't get in at all. No moisture ingress means that the important heat exchange surfaces will never freeze.
Getting rid of frost buildup makes energy-intensive defrost cycles unnecessary, which gets rid of the main cause of temperature excursions . This leads to constant, rock-solid thermal stability, which is the most important part of keeping plasma quality stable.
There are a lot of operational benefits. Energy use goes down because power is no longer sent to heating coils. Mechanical reliability goes up because parts work under steady-state conditions, which means they don't have to deal with the stress of repeated heating and cooling. Air-cycle refrigeration fixes a lot of core plasma storage problems by dealing with the root causes of moisture, ice, and instability all at once.
How Dependable Plasma Storage Is Supported by MIRAI INTEX
MIRAI INTEX is focused on production of ultra low temperature systems. It develops advanced cooling technologies for mission-critical medical cold storage, including ultra-low temperature freezing and blood plasma storage. These systems solve common plasma storage problems.
Some of its applications are blood plasma freezing and frozen plasma storage systems. These systems eliminates moisture-related ice formation and defrost cycles, ensuring superior temperature stability for plasma quality preservation. This first-principles engineering ensures reliability is built into the system from inception, safeguarding plasma integrity from collection to final therapeutic use.
MIRAI X CRYO Plasma Storage Systems
The MIRAI X CRYO systems is an example of this way of thinking about engineering. They are not just chillers; they are complete systems. Designed for the most demanding frozen plasma storage needs, these systems offer precise temperature control over a wide range from +90 to -160 degrees Celsius within ±0.5°C even when the load changes.
The systems use oil-free compressor technology, which means that there is no chance of hydrocarbon contamination at all. This is very important for plasma storage that will be used for fractionation or direct clinical use. The MIRAI X CRYO series is made with materials that are safe for use in pharmaceuticals and is designed to fit perfectly into controlled environments. It is a complex solution that was made to meet the high standards for reliability and documentation that modern blood plasma storage operations need.
Economic and Operational Advantages
Energy use goes down a lot when you get rid of defrost cycles and use efficient compression principles. There is no ice buildup, and the refrigerant (air) is strong and simple to use, which means less maintenance is needed and lower lifetime service costs. Improved reliability means less downtime and longer equipment life, which lowers the total cost of ownership for frozen plasma storage by a lot, while also providing better protection for the product.
Environmental and Regulatory Factors
The outside world is affecting decisions about plasma storage. The EU F-Gas regulation impact and other similar efforts around the world are making it harder and harder to use high-GWP HFC refrigerants. Facilities must now consider how well their capital equipment meets environmental standards and how well it works. Using eco-friendly technologies is in line with a company's ESG (Environmental, Social, and Governance) goals and lowers the risk of owning assets that don't meet those goals. Choosing a system with a sustainable, regulation-proof refrigeration platform is no longer an option for mission-critical plasma storage; it is now a strategic necessity for long-term operational security.
Conclusion: Developing a Secure and Dependable Plasma Storage System
Choosing the right technology is an important part of making sure the plasma storage supply chain is safe. Facilities can go from reactive maintenance to proactive prevention by learning how temperature instability, ice formation, and old refrigerants can have a big effect. The best way to move forward is to combine advanced, purpose-built systems that make sure freezing is even, stop defrost-related excursions, and use eco-friendly refrigerants like air. There are a lot of reference projects that prove its reliability and future-proof. This method guarantees the highest standards of plasma quality, compliance with all regulations, easy service and support, operational reliability, and long-term cost-effectiveness.

