Project Reference: PharmaBlastFreeze – Advanced Cryogenic Freezing System

Overview
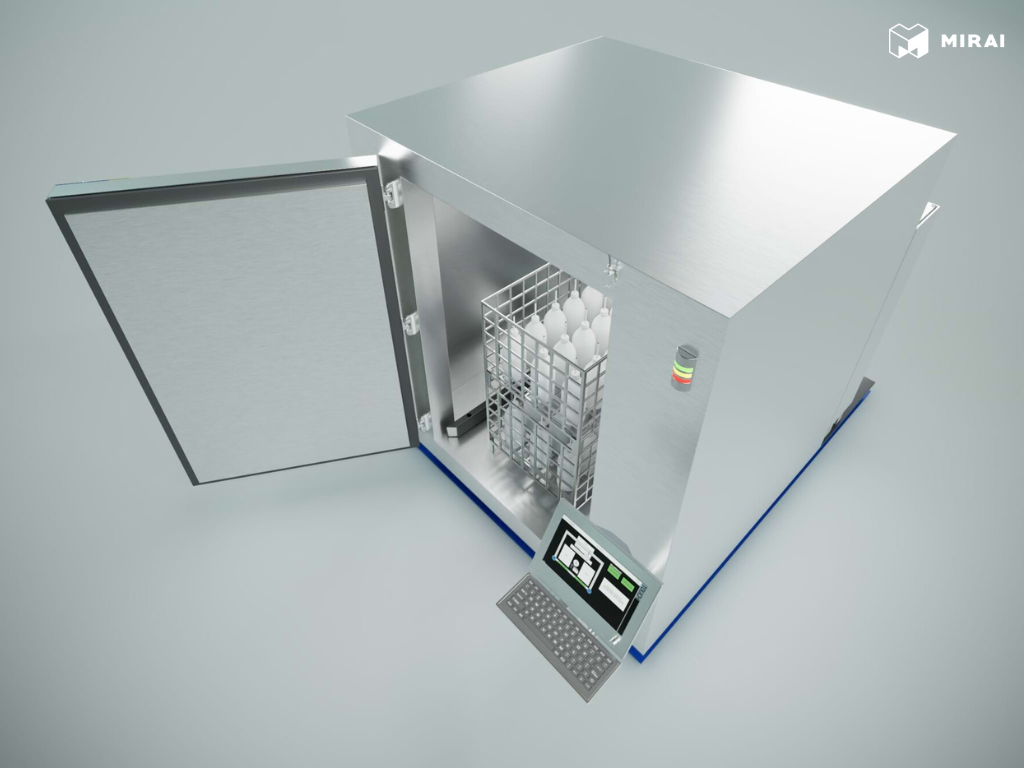
The PharmaBlastFreeze project introduces a state-of-the-art cryogenic freezing system engineered by Refolution Gmbh to meet the rigorous demands of the pharmaceutical and biotechnology industries. This system is designed for the controlled and homogeneous freezing of products stored in pallets and large containers, critical for maintaining product integrity and quality during low-temperature storage and transport. Leveraging a compact design, the PharmaBlastFreeze offers universal compatibility with various packaging materials, including bag and bottle systems, making it a versatile solution for diverse product forms.
Technical Specifications and Innovation
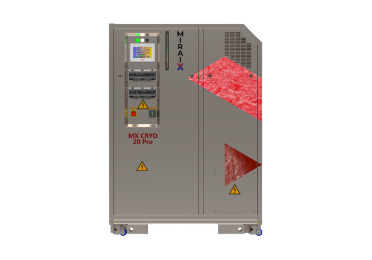
Refrigeration Technology – Powered by MIRAI X CRYO 20
At the core of the PharmaBlastFreeze's ultra-low temperature capability is the MIRAI X CRYO 20 water-cooled cold air chiller. This specific model from Mirai Intex utilizes natural air (R729) as the sole refrigerant, representing a significant advancement in environmentally responsible cooling. This proprietary air-cycle technology operates in a closed loop, completely eliminating the need for traditional F-gases and their associated global warming potential (GWP 0) and ozone depletion potential (ODP 0).
The MIRAI X CRYO 20 employs an advanced turbo-compressor (water-cooled), a key component developed in-house by Mirai Intex. This oil-free design, featuring air bearings, significantly reduces wear and tear, enhancing reliability and minimizing maintenance requirements. The technology operates on the principle of air compression and expansion cycles, where energy recovered during expansion is transferred back to the compressor, leading to high energy efficiency.
Performance Parameters of the MIRAI X CRYO 20
The integration of the MIRAI X CRYO 20 enables the PharmaBlastFreeze to achieve a flexible chamber temperature range of +90 °C to -160 °C. The system maintains exceptional precision with a temperature accuracy of ± 0.5 °C under changing load conditions, and even higher accuracy at idle.
Key operational characteristics of the MIRAI X CRYO 20 that contribute to the PharmaBlastFreeze's performance include:
- Rated Motor Power: 20 kW
- Refrigeration Capacity: Up to 7.7 kW (at a heat transfer fluid temperature of -80°C and cooling water temperature of 10°C, typical for this model in its range).
- Noise Level: Max. 70 dB, indicating quiet operation for an industrial chiller of this capacity.
- Compact Dimensions: The chiller itself has a footprint of approximately 180 x134x99 cm, contributing to the PharmaBlastFreeze's overall minimal space requirement.
System Architecture and Compliance
Constructed entirely from stainless steel, the interior of the PharmaBlastFreeze ensures ease of cleaning and adherence to the stringent hygiene requirements of pharmaceutical environments. Its compact dimensions (approx. 3465 x 2500 x 2720 mm) and integrated refrigeration system behind the product chamber optimize facility footprint and allow for flexible installation in both classified and non-classified areas.
Process control is managed by the "HOF Freeze Viewer Professional" visualization system, which is fully compliant with FDA 21 CFR Part 11 regulations. This automation suite facilitates comprehensive data acquisition, including batch reports and temperature curves, ensuring full traceability and regulatory compliance. The system supports the generation of detailed process reports and seamless integration with higher-level management systems.


Operational Advantages
- Environmental Sustainability: Utilizes R729 natural air refrigerant, aligning with future-proof environmental regulations and eliminating F-gas related concerns.
- Enhanced Safety Profile: Absence of hazardous refrigerants and an oil-free system design eliminate associated safety risks, simplifying installation and operation.
- Optimal Temperature Control: Provides precise and flexible temperature management across a wide cryogenic range (+90 °C to -160 °C), adaptable to various product specifications.
- High Throughput & Uniformity: Rapid freezing capabilities combined with superior air circulation ensure product consistency and efficiency.
- Minimized Footprint: The compact nature of the MIRAI X CRYO 20 contributes to the overall integrated design, maximizing valuable production space.
- Robust Reliability: Engineered for durability and fail-safe operation, thanks to oil-free, air-bearing technology and automatic restart capabilities after power interruptions.
- Integrated Project Support: Mirai Intex (in collaboration with HOF) provides end-to-end support from planning and manufacturing to commissioning, training, and ongoing service and maintenance, ensuring optimal system performance.
.png)